Patent watch: ELECTROLYTE COMPOSITIONS FOR BATTERIES USING SULPHUR OR SULPHUR COMPOUNDS
There are disclosed electrolytes comprising solutions of lithium salts with large anions in polar aprotic solvents with a particular concentration of background salts. The concentration of the background salts is selected to be equal or close to the concentration of a saturated solution of these salts in the aprotic solvents used. The electrolytes disclosed can be used in chemical sources of electric energy such as secondary (rechargeable) cells and batteries comprising sulphur-based positive active materials. The use of such electrolytes increases cycling efficiency and cycle life of the cells and batteries.
Inventors: Vladimir KOLOSNITSYN, Elena KARASEVA
Application number: 13/153,157 Publication number: US 2011/0236766 A1 Filing date: Jun 3, 2011

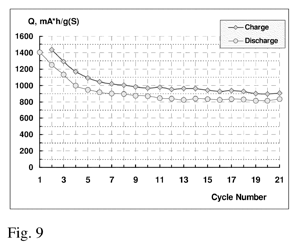
Fig. 1 Charge/discharge capacity fade of standard Li-S cell
Fig. 9 Charge/discharge capacity fade of Li-S cell with a saturated electrolyte solution as 1.7M of LiClO4 in methylpropylsulfone @ 0.25C ch/dch
Fast capacity fade and relatively low cycling efficiency are the main problems encountered when designing lithium-sulphur batteries. Irreversible transfer of sulphur from the positive electrode (cathode) to the surface of the negative electrode (anode) and its accumulation there in the form of lithium sulphide or disulphide is one of the major reasons for capacity fade during cycling of lithium-sulphur cells. The low cycling efficiency of lithium-sulphur batteries is caused by the reversible transfer of sulphur in the middle of the charge and discharge process. This transfer results in what is known as the sulphide cycle, i.e. the energy transfer inside the battery (in its internal circuit).
Elemental sulphur and the end products of sulphur reduction (lithium sulphide or disulphide) are known to be poorly soluble in most organic solvents. In contrast, lithium polysulphides (intermediate forms produced during the reduction of elemental sulphur or during oxidation of lithium sulphide and disulphide) are well soluble in many organic solvents.
Lithium polysulphides may be present in electrolyte systems in three forms: molecular, mono-anionic, and di-
anionic. Hence sulphur in the electrolyte can be transferred either in molecular (neutral) or in ionic (anionic) form. The diffusion of elemental sulphur and non-dissociated lithium polysulphides dissolved in the electrolyte contributes to the molecular transfer of sulphur. The diffusion and electromigration of the mono- and di-anions of polysulphides, as well as sulphur anion-radicals, contributes to the ionic form of sulphur transfer. The existence of two mechanisms increases the overall sulphur transfer.
When forming the electrolyte compositions of embodiments of the present invention, the following considerations may be taken into account:
[0054] 1) The electrolyte composition should comprise a non-aqueous aprotic solvent, lithium or another alkali metal salt and optional modifying additives.
[0055] 2) Said salt can be an individual salt or a number of different salts.
[0056] 3) Said salt or number of salts are dissolved in an individual aprotic polar solvent or a mixture of solvents.
[0057] 4) Said electrolyte composition should be chosen in a way that the concentration of the lithium salt or the mixture of salts is equal (or close) to the concentration of a saturated solution of the salt or salts used in the solvent or mixture of solvents.
Comments