Patent watch: SEPARATOR INCLUDING POROUS COATING LAYER AND ELECTROCHEMICAL DEVICE
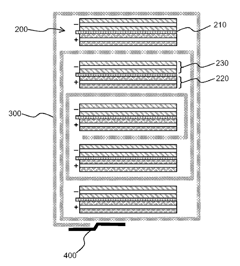
Disclosed is a separator. The separator includes a planar non-woven fabric substrate having a plurality of pores, and a porous coating layer formed on at least one surface of the non-woven fabric substrate. The porous coating layer is composed of a mixture of filler particles and a binder polymer. The filler particles include conductive positive temperature coefficient (PTC) particles composed of a mixture of conductive particles and a low melting point resin having a melting point lower than that of the non-woven fabric substrate. Due to the presence of the conductive PTC particles, the porous coating layer can be imparted with a shutdown function against thermal runaway. In addition, the porous coating layer exhibits appropriate electrical conductivity. Therefore, the separator is suitable for use in a high-capacity electrochemical device. Inventors: Pil-Kyu Park, Jong-Hun Kim, Soon-Ho Ahn, Je-Young Kim Original Assignee: LG CHEM, LTD. Application number: 13/173,902; Publication